Clinical Trial Information
The planned research consists of three distinct clinical trials that focus on three different hospital patient groups and environments. The first study targets areas associated with the sickest patients - those in intensive care units. The proposed second study examines the effect of copper touch surfaces in areas with a less intensely ill group (e.g., cancer patients). The final proposed study evaluates whether the intervention is also efficacious for areas associated with "routine" patients - those hospitalized on a regular medical ward (e.g., gastrointestinal care unit).
Each of the three clinical trials will employ the same methodology for determining microbial burden levels for the selected environment as well as for determining transmission rates from inanimate touch surfaces to patients. Specific methods for each phase of the trials are described below. The three clinical trals are being supported by the US Army Medical Research and Material Command (USAMRMC) - Contract W81XWH-07-C-0053.
Phase I - COMPLETED FOR THE FIRST CLINICAL TRIAL
Determine baseline microbial bioload in a clinical arena and identify critical surfaces that will be replaced with copper alloy touch surfaces.
This first phase provides for the necessary research to enable the team to determine baseline microbial burdens of the monitored microbes on surfaces within the selected patient care setting. The results will establish the control values from which efficacy of the intervention will be based. Although there will be human contact with surfaces in this phase, only the surfaces themselves will be measured. This phase also includes the identification and selection of appropriate touch surfaces to be reconfigured with copper alloys. It also includes the fabrication and installation of the selected items.
This phase has been completed for the first clinical trial. The results were presented at the Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) in Washington, D.C., in October 2008. They are summarized below. The complete poster is also available for download.
Download Microbial Burden of Objects in ICU Rooms [complete PDF - 196 KB]
Results:
1760 objects in 160 rooms were sampled:
- 660 objects in 60 rooms at hospitals A & B
- 440 objects in 40 rooms in Hospital C
The mean Microbial Burden (MB) of the room was 16,885 cfu per 100 sq cm (Figure 1).
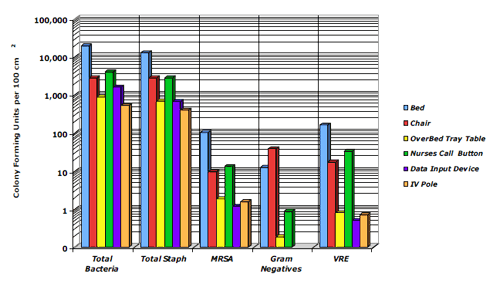 Figure 1. Mean MB of Objects in Patient ICU Rooms
Figure 1. Mean MB of Objects in Patient ICU Rooms- Bed rails had the highest mean MB (Figure 2) comprising:
- 58% of the MB in Hospital A
- 49% of the MB in Hospital B
- 89% of the MB in Hospital C
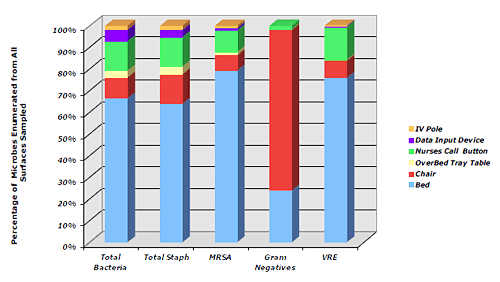 Figure 2. Distribution of MB Among Objects in Patient ICU Rooms
Figure 2. Distribution of MB Among Objects in Patient ICU RoomsObjects in close proximity to the patient had significantly higher mean MBs compared to other objects in the room:
- Bed rails, call button, chair at Hospital A (p 0.04 to <0.0001)
- Bed rails, call button, chair, and data device at Hospital B (p 0.01 to <0.0001)
- Bed rails and chair at Hospital C (p 0.03 to <0.0002)
Staphylococcus was the predominant organism isolated from each object and each room comprising:
- 65% of the mean MB in hospital A
- 73% in Hospital B
- 60% in Hospital C
MRSA, VRE and gram negatives were isolated but were generally <5% of the mean MB.
Phase II - COMPLETED FOR THE SECOND CLINICAL TRIAL
Measure and compare the impact of copper alloy touch surfaces for its ability to reduce the levels of harmful microbes in a clinical area.
The results of Phase II testing at the Medical University of South Carolina are presented below. The results from the other 2 institutions will be presented here after they have been published in a peer-reviewed forum.
Copper alloy touch surfaces were installed in three randomly selected Medical Intensive Care Unit (MICU) rooms, such that comparative testing could be undertaken for both copper and non-copper control surfaces to determine whether there was any significant reduction and difference in microbial bioload (MB) reduction on each of the surfaces in each of those areas.
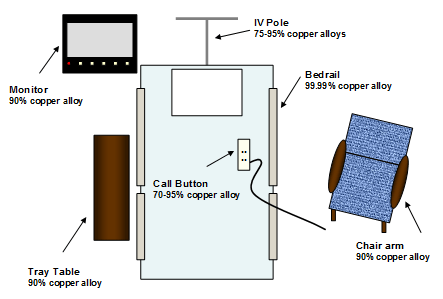 Figure 1: Schematic of objects in patient ICU rooms
Figure 1: Schematic of objects in patient ICU roomsThe objects involved bed rails, tray tables, chair arms, nurse's call buttons, monitors and IV poles. The objects were sampled by the sterile wipe technique weekly for nine weeks and the microbial burden for each object and each room (sum of all objects) was determined as colony forming units (cfu)/100 cm2. The efficacy of copper was calculated as the difference in mean MB between the copper and non-copper objects and rooms (environmental cleaning regimens did not change over the study period). The Kruskall-Wallis test was used to test all rooms.
DOWNLOAD Pilot Study to Determine the Effectiveness of Copper in Reducing the Microbial Burden of Objects in Rooms of Intensive Care Units (ICU) Patients [Complete PDF - 688 KB].
Results:
282 copper objects in 32 rooms and 288 non-copper objects in 27 rooms were sampled. Copper was effective in significantly reducing the total mean MB of the patient ICU room by 87.4%. Copper was also effective in reducing the MB on four of the six objects. They showed no reduction in the mean MB on the tray tables or monitors
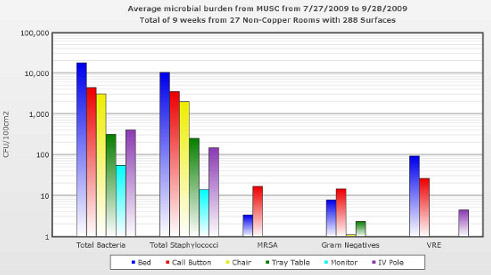 Figure 2: Mean microbial burden of objects in MICU control rooms.
Figure 2: Mean microbial burden of objects in MICU control rooms.Staphylococcus was the predominant organism isolated from each individual object, whether it was copper or on-copper and comprised 78.7% of the mean MB of copper rooms and 55.5% of non-copper rooms. Of note, MRSA and VRE were frequently isolated from non-copper rooms, but were never isolated from copper objects.
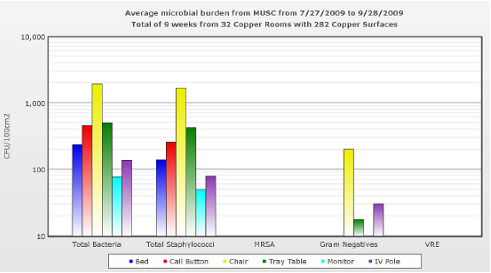 Figure 3: Mean microbial burden of copper objects in MICU rooms.
Figure 3: Mean microbial burden of copper objects in MICU rooms.Conclusion:
- Objects found in ICU rooms can serve as a reservoir for the spread of bacteria, especially staphylococci, to patients, healthcare workers and visitors
- Copper significantly reduced the total mean MB by 87.4% in ICU patient care rooms and on many individual objects within the rooms
- Significant reduction was seen when bed rails and call buttons were copper, while these items accounted for 83.3% of the total mean MB in non-copper rooms
These results were presented at a poster session in the 2010 Fifth Decennial International Conference on Healthcare-Associated Infections, in Atlanta, Georgia, March 18-22. This conference was co-organized by the U.S. Centers for Disease Control and Prevention (CDC), The Society of Healthcare Epidemiologists of America (SHEA), The Association for Professionals in Infection Control and Epidemiology (APIC), and the Infectious Diseases Society of America (IDSA).
Phase III
Measure rate of acquisition and transfer of monitored microorganisms from touch surfaces to patients and from patients to touch surfaces.
This phase will establish the effectiveness of copper alloy touch surfaces in the selected clinical areas for their ability to prevent the transfer of the monitored microbes from touch surfaces to patients and from patients to touch surfaces. Eligible subjects will be randomized to coppered and non-coppered rooms and then followed until they are discharged from the assigned room. Transmission will be determined by the evaluation of nasal and perirectal swabs collected from randomized patients during the period of their stay. This phase also includes the preparation of a manuscript and submission of the clinical trial results to a leading, peer-reviewed medical journal.